C7h6o3 C4h6o3 C9h8o4 C2h4o2 2024 Usa
C7h6o3 C4h6o3 C9h8o4 C2h4o2 2024 Usa. Balance the following chemical equation (if necessary) for the synthesis of aspirin from salicylic acid and acetic anhydride shown below: This method uses algebraic equations to find the correct.
C7h6o3 + c4h6o3 = c9h8o4*h2o is a synthesis reaction where seven moles of protocatechualdehyde [c 7 h 6 o 3] and eight moles of acetic anhydride [c 4 h 6 o 3]. Now, both sides have 4 h atoms and 2 o atoms.
C7h6o3 C4h6o3 C9h8o4 C2h4o2 2024 Usa Images References :
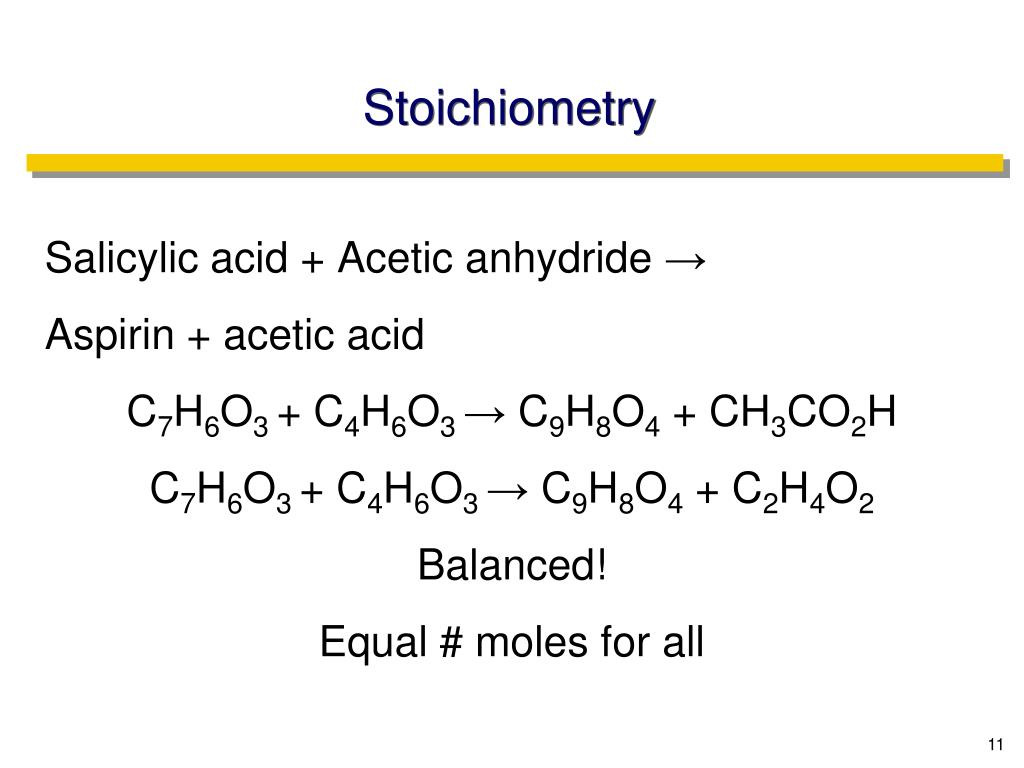 Source: www.slideserve.com
Source: www.slideserve.com
PPT CHAPTER 3B Stoichiometry PowerPoint Presentation, free download, Balance the following chemical equation (if necessary) for the synthesis of aspirin from salicylic acid and acetic anhydride shown below:
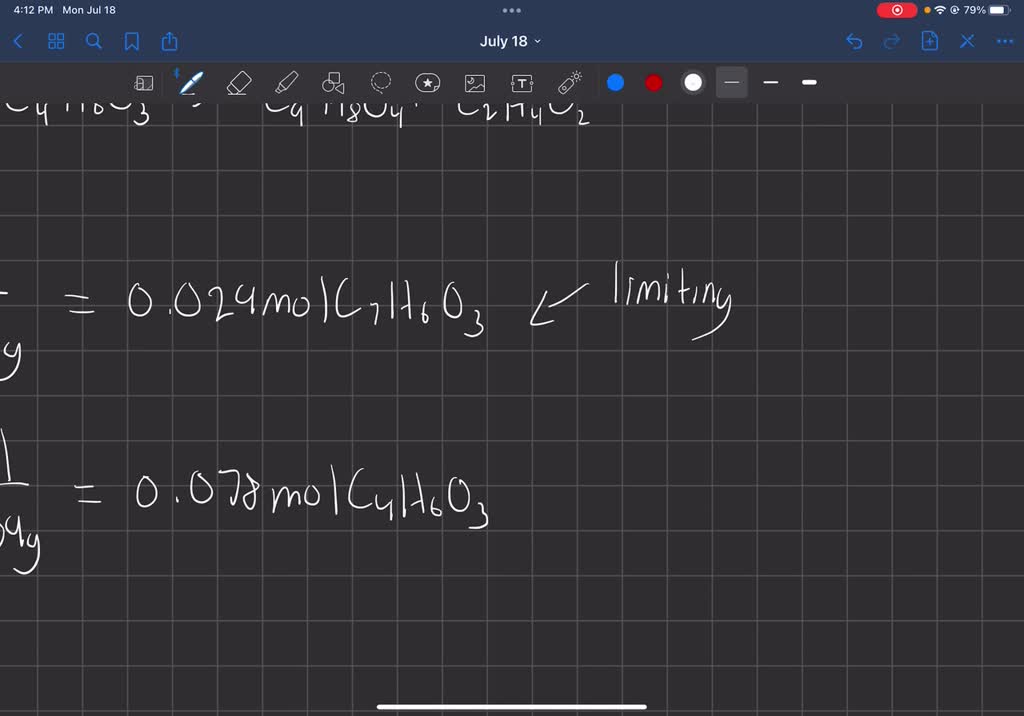 Source: www.numerade.com
Source: www.numerade.com
SOLVED Aspirin (C9H8O4) is prepared by heating salicylic acid (C7H6O3, Now, both sides have 4 h atoms and 2 o atoms.
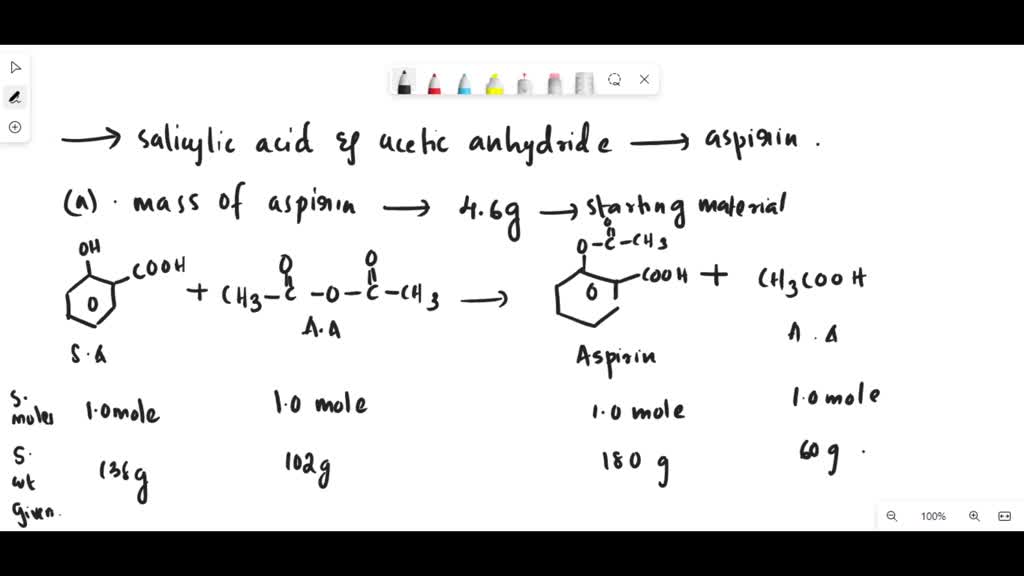 Source: www.numerade.com
Source: www.numerade.com
SOLVED The reaction of salicylic acid, C7H6O3, with acetic anhydride, C7h6o3 + c4h6o3 = c9h8o4*h2o is a synthesis reaction where seven moles of protocatechualdehyde [c 7 h 6 o 3] and eight moles of acetic anhydride [c 4 h 6 o 3].
 Source: www.numerade.com
Source: www.numerade.com
SOLVED 16.C7H6O3 C4H6O3 C9H8O4 C2HO2 salicylic acetic acid anhydride, Now, both sides have 4 h atoms and 2 o atoms.
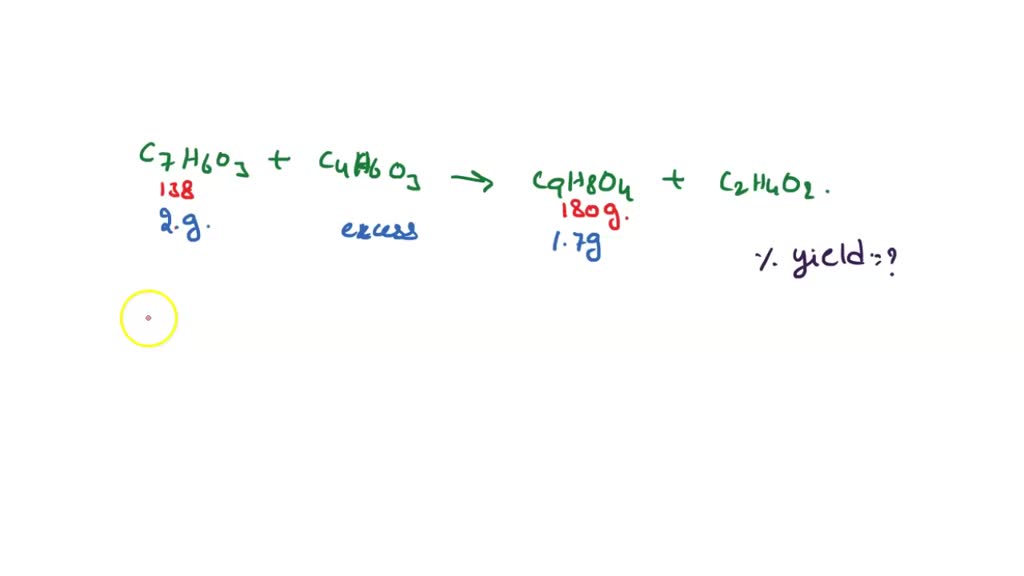 Source: www.numerade.com
Source: www.numerade.com
SOLVED If a student isolated 2.27 g of aspirin, C9H8O4, from the, Balance the following chemical equation (if necessary) for the synthesis of aspirin from salicylic acid and acetic anhydride shown below:
 Source: www.chegg.com
Source: www.chegg.com
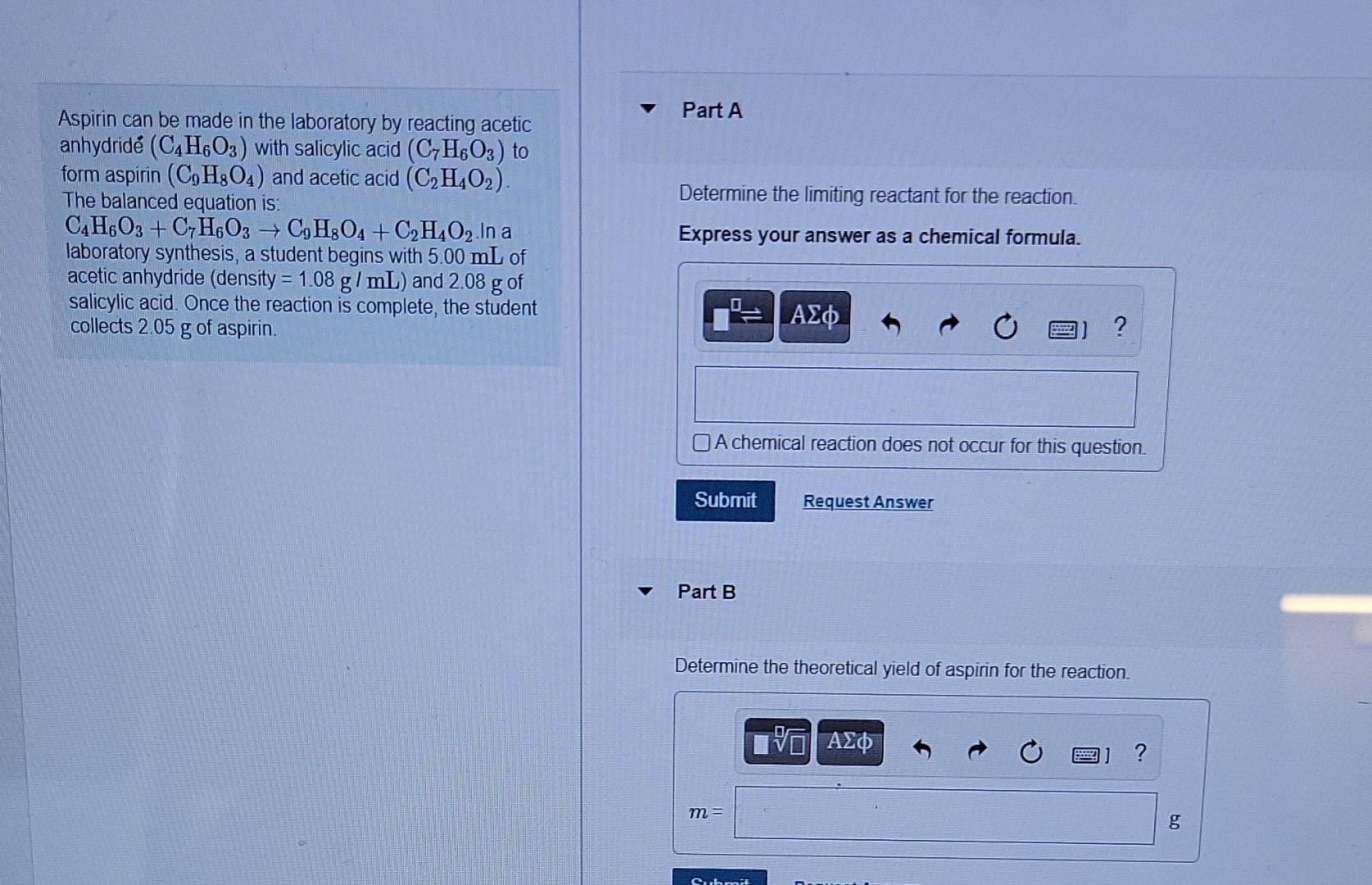
Solved (7) Aspirin (C9H8O4, molar mass = 180.157 g/mol) is, Balance the following chemical equation (if necessary) for the synthesis of aspirin from salicylic acid and acetic anhydride shown below:
 Source: www.chegg.com
Source: www.chegg.com
Solved Part A Determine the limiting reactant for the, Balance the reaction of c7h6o3 + c4h6o3 = c9h8o4 + c2h4o3 using this chemical equation balancer!
 Source: www.numerade.com
Source: www.numerade.com
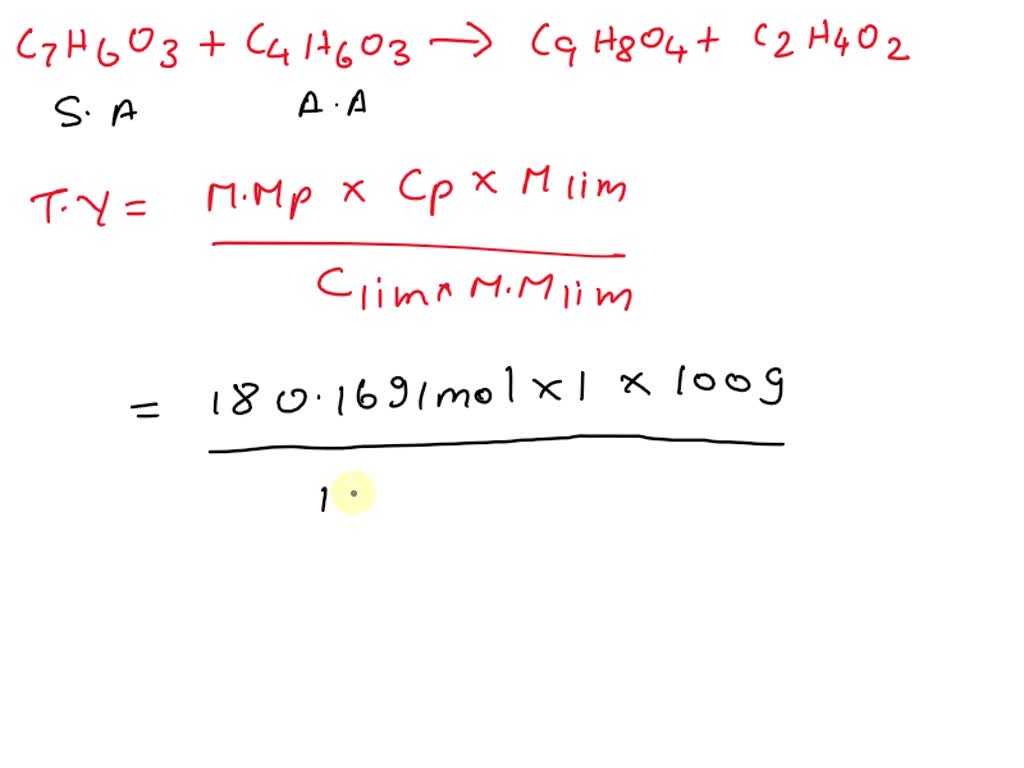
SOLVED Student prepared aspirin (C7H6O3) in a laboratory experiment, Aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin (c9h8o4) and acetic acid (c2h4o2).
 Source: www.numerade.com
Source: www.numerade.com
SOLVED The chemical reaction of 100 g of salicylic acid, C7H6O3, with, C7h6o3 + c4h6o3 = c9h8o4*c2h4o2 is a synthesis reaction where one mole of protocatechualdehyde [c 7 h 6 o 3] and one mole of acetic anhydride [c 4 h 6 o 3] combine.
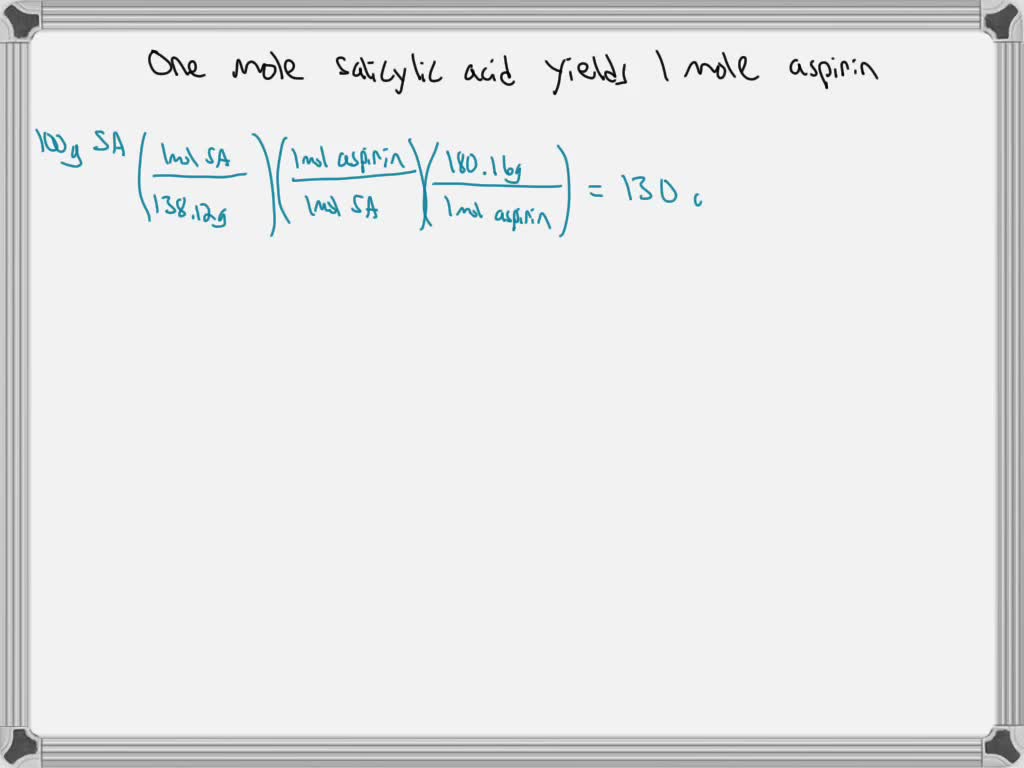 Source: www.numerade.com
Source: www.numerade.com
SOLVED The chemical reaction of 100 g of salicylic acid, C7H6O3, with, Balance the reaction of c7h6o3 + c4h603 = c9h804 + h2o using this chemical equation balancer!
Posted in 2024